

The ubiquitin system controls virtually all aspects of the cell. E3 ligase enzymes conjugate the small protein ubiquitin to protein substrates, which marks them for degradation and serves as a powerful regulatory mechanism of the cell. Dysfunction of the ubiquitin system is a hallmark of diseases such as cancer and neurodegeneration and its modulation has huge therapeutic potential.
Our lab develops and applies tools to explore the ubiquitin system in an unbiased and holistic way. Excitingly, this has allowed us to uncover an unexplored dimension of ubiquitin biology. For example, we have shown that the mammalian protein MYCBP2 is a new class of E3 ligase with a paradigm-redefining ubiquitin transfer mechanism. We have also shown that it conjugates ubiquitin using entirely different "non-lysine" linkage chemistry and this positively regulates programmed axon death. We have also identified a class of deubiquitinating enzymes that selectively reverse non-lysine ubiquitination, revealing that it is a sophisticated and regulated arm of the ubiquitin system.
To investigate this new dawn of ubiquitin biology we are using multidisciplinary approaches to unravel the ubiquitin transfer mechanisms of novel classes of E3 ligase, how they are activated, and how they recognise their substrates. We are also developing and applying tailored technologies to understand the molecular basis and function of non-lysine ubiquitination.
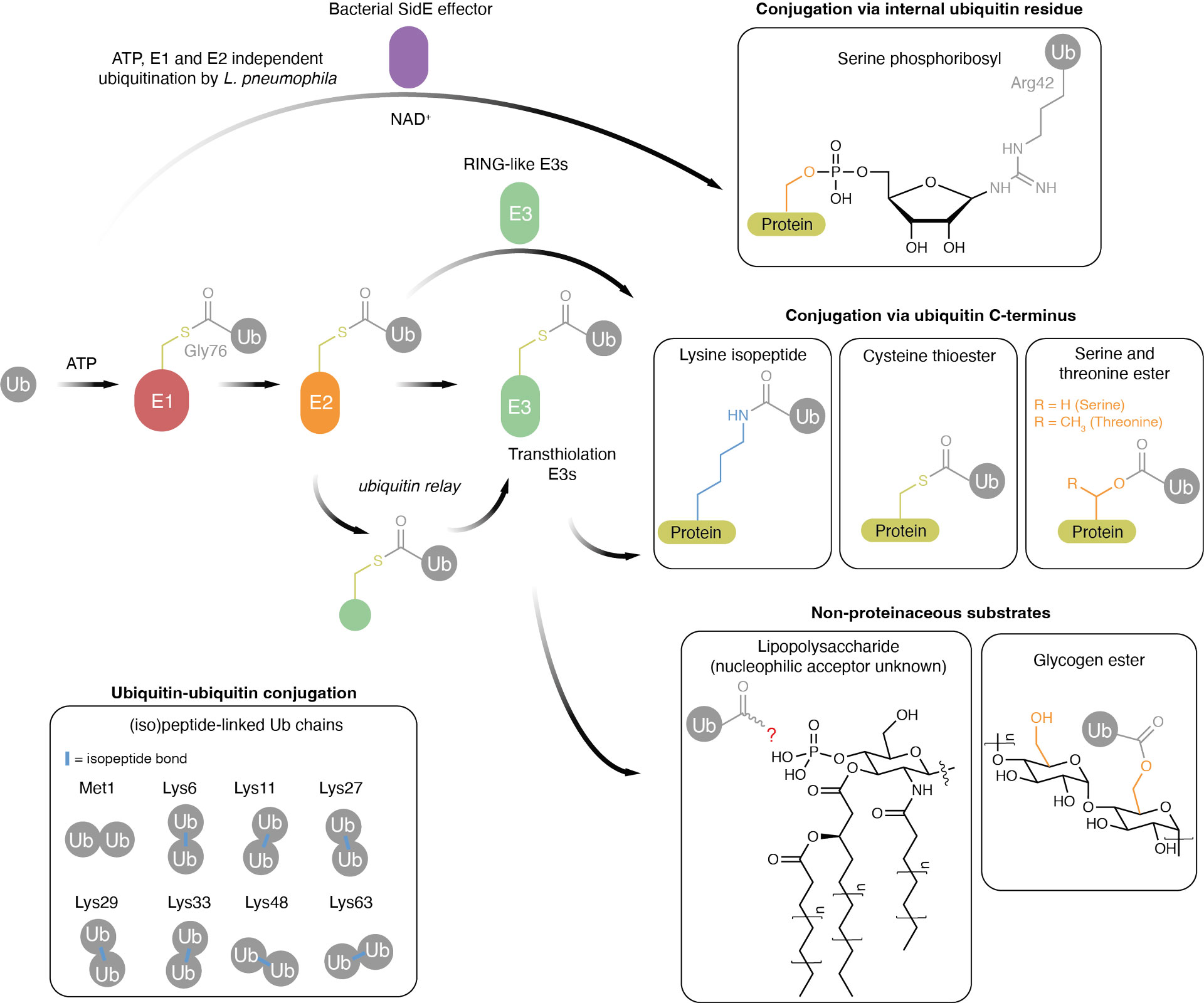
Figure 1. The growing world of ubiquitin conjugation . Ubiquitin is initially adenylated at its C-terminus in an ATP-dependent reaction. It is then transferred to a catalytic cysteine residue in E1 forming a thioester-linked conjugate (E1~Ub). In humans there are 2 ubiquitin E1 activating enzymes. Next, one of ~35 E2s can be recruited that undergo transthiolation with E1~Ub. The charged E2 (E2~Ub) can then transfer Ub to a HECT/RBR E3 ligase (E3~Ub). This activity is regulated and dysregulated in a number of diseases. Thousands of proteins are ubiquitinated in cells.
Copyright © 2022 · All Rights Reserved · Virdee Lab
MRC Protein Phosphorylation and Ubiquitylation Unit
University of Dundee
UK