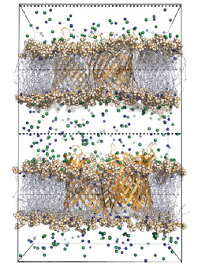
Potassium channels, amongst other ion channels, are amongst the most important biological systems involved in signalling across the membrane, for instance in nerve impulses. We have developed methods to computationally predict the ionic conductance of these channels and at the same time obtain an accurate, atomistic view of permeation mechanisms. This is important, for instance, to understand how cardiac ion channels function to control heartbeat - and how their malfunction can lead to arrhythmia and sudden death. This mechanistic insight can help in the design of anti-arrhythmic drugs, and also in avoiding undesired interactions with these channels.
Related publications:
The world is running out of antibiotics at increasing pace. Many bacterial strains have become multi-resistant over the last decades. Some bacterial infections have even become impossible to treat with traditional antibiotics. In our research, we look at the molecular basis for the development of resistance and we search for new antibiotics that are less likely to lead to resistance.
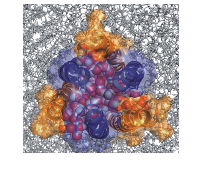
Dermcidin is a member of the class of peptidic antimicrobials, produced by all higher organisms to defend themselves against infections. It is produced in sweat glands and on the human skin, and acts as a medium-efficacy broad spectrum antibiotic. As part of a multi-national collaboration, we have shown that the newly solved assembly structure of dermcidin conducts ions through bacterial membranes with a unique mechanism involving sideway ion entry. The conductance predicted by us is in quantitative agreement with experiment.
Related publications:
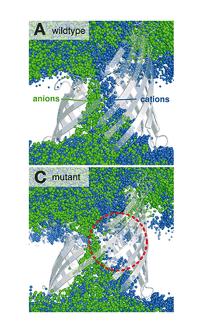
Neisseria meningitidis is the main cause for bacterial meningitis. It is a Gram-negative bacterium whose outer membrane contains porins, protein gates that enable it to take up polar nutrients. The same pores have to be passed by antibiotic molecules diffusing into the organism. So small mutations in these pores are sufficient to contribute to the development of resistance. Shown here is the effect of a single mutation on the pathways of anions and cations in the Neisserial channel PorB. In the past, we also intensively studied other bacterial porins such as Omp32.
Related publications:
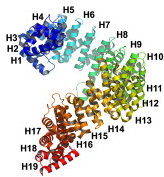
Helical tandem repeat proteins are optimised systems for strong protein-protein interactions which have an enormous degrees of flexibility and elasticity. Recently, we have put a focus on the nuclear export receptor CRM1.
The nucleus is the defining compartment of eukaryotic cells. Translation of nuclear genes by the cytoplasmic ribosomal machinery requires a finely controlled transport process between the cytoplasm and the nucleus, in which proteins and RNA traverse nuclear pores with the help of transport factors. Nucleocytoplasmic transport is central to the expression of genes, and thus also plays an important role in the development of cancer. Defects in nucleocytoplasmic transport have therefore been detected in a wide variety of cancer cells. The principal cellular nuclear export mechanism is mediated by the transport factor CRM1. It is involved in particularly important pathways of cancer initiation and progression. CRM1 interacts with nuclear proteins tagged for export by a nuclear export signal (NES), and shuttles them out of the nucleus. Malignant cells have been shown to exhibit mislocalisation of multiple tumour suppressor proteins from the nucleus, including that of p53, which can be caused by CRM1 overexpression. CRM1 has thus recently been validated as an important cancer target. We have recently characterised large-scale structural transitions of CRM1 between its free and NES-cargo bound states using crystallography, electron microscopy, and molecular dynamics simulations (Monecke et al., PNAS 2013 and Dölker et al., Structure 2013). Strikingly, the degree of opening of the NES-binding cleft, and thus its NES-cargo binding capacity, is highly dependent on allosteric movements caused by molecular interactions at distant sections of CRM1. In particular, the crystal structure of the open state of CRM1 shows that the C-terminal alpha-helix of CRM1 can prop the whole molecule open and thereby close the NES cleft.
Related publications: